- red photon energy calculation, in J and eV (see 4/25 notes). Visible light photon energy ranges from 1.6-3.1 eV.
- "electron-Volt" eV is a useful unit of energy for photons. Photons can come from atomic or chemical energy level transitions, and these are associated with potential energy changes of the order of volts, and they can be induced by or can produce visible photons. This is why visible photon energies are in this range.
- fluorescent lamps. The important mercury transition to its ground state is is a photon of wavelength 254 nm, and energy 4.9 eV. This is beyond the visible, in the ultraviolet (UV) range. It is absorbed by glass. Isn't that remarkable, that a photon quite similar to visible ones is absorbed, while visible light is transmitted. This is because the visible photons don't have adequate energy to induce electronic transitions in the glass, but UV ones do. This in itself illustrates quantum behavior. Otherwise, I discussed aspects of the fluorescent lamp more or less as in the textbook. Note that with the diffraction grating last week we looked at the fluorescent lamps in the room and saw that although they appear white, their spectrum is NOT full of all colors, but rather has content something like this:
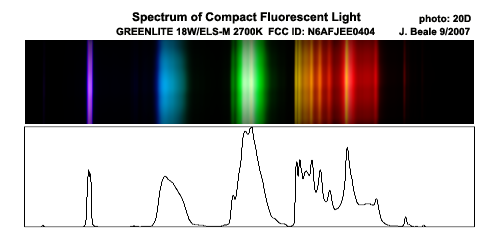
This combination of frequencies produces in the three cone types of our retina a similar response to what a full spectrum of white light would produce, illustrating again in what sense we are all color blind: we can't distinguish light with some very different spectral content.
LASER light: characterized by the fact that all the photons have nearly the same:
i) wavelength
ii) direction
iii) phase (they oscillate in step)
How to produce laser light: first we must take note of the fact that photons can be created in two ways when an atomic transition occurs frmo a higher to a lower energy level: 1) spontaneous emission, and 2) induced, or stimulated emission. Spontaneous emission occurs just because the accelerating electron radiates. It happens at a random time and the photon can come out in any direction. Induced emission, by contrast, occurs when an incoming photon interacts with the excited electron. That interaction shakes the electron. If the incoming photon has just the right frequency so that its energy matches the transition energy, then it can induce the excited electron to make a transition, and in the process the new photon that is generated is more likely to be an identical copy of the incoming photon than anything else. So induced emission can "clone" the photon.