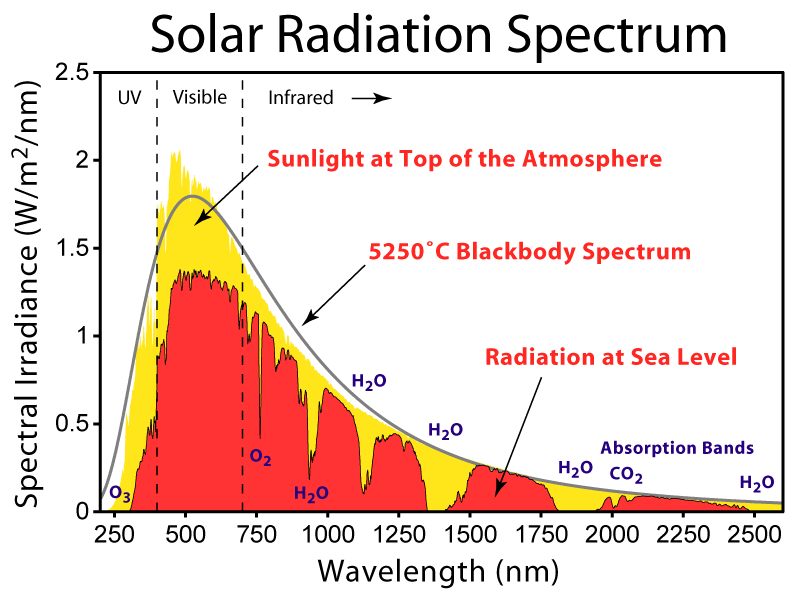
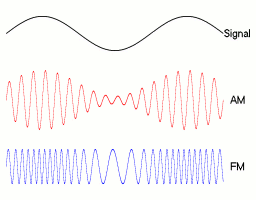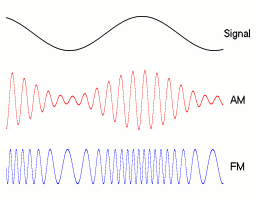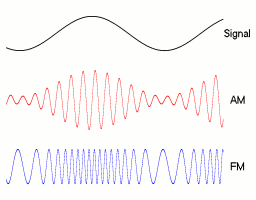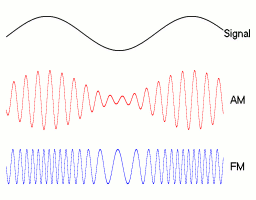
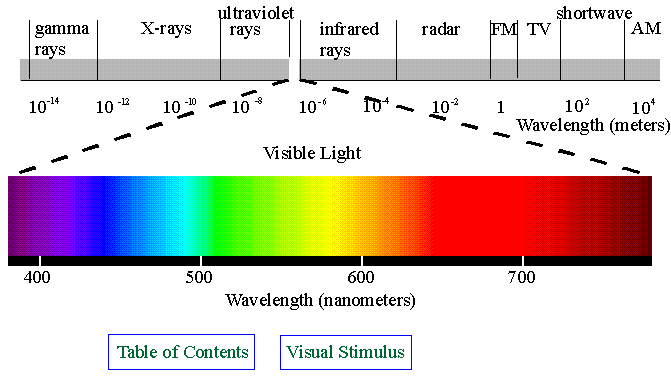
a linear polarizer in back and a layer that converts circular to linear polarization in front. You can demonstrate
the fact that the front and back are different by holding up the glasses in front of an LCD display (which emits
linearly polarized light). If the back of the glasses faces the display, then by rotating the lens you can block
out all the light since the polarizers are crossed. If the front of the glasses faces the display then you can rotate
all you want but you'll never block all the light. (When held at a certain angle the light is bluish and when
held perpendicular to that it is yellowish. I don't yet know why this is... I asked the company and they
told me it is considered proprietary information.)
- Color perception: as described on pp. 454-455. Besides that, I noted the following:
- the names of the cone cells, "red", "geen" and "blue", only indicate the location of their peak sensitivity;
they are sensitive to many colors of the spectrum. The "red" cone even has some sensitivity to blue and
indigo.
- we are much less sensitive to violet than to blue light.
- in a sense, we are all color blind: there is an infinite variety of different mixtures of colors of the spectrum,
and our eyes filter all that into some combination of the three cone cell responses. In fact, the overall response
level is just "brightness", so there are actually two color variations we sense. You can think of these as the
proportion of "red" vs. total cone response, and the proportion of "green" vs. total cone response. The
proportion of "blue" is whatever is left so is not an independent feature. This two-dimensional color space
is illustrated here:
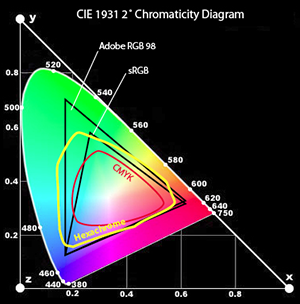
(Diagram taken from here: http://www.digitalfieldguide.com/blog/1296)
The spectral colors are around the boundary of this shape, labeled by their wavelength in nanometers. The line
of purples along the bottom consists of colors that are not in the spectrum. The different shapes and triangles inside
show what region can be faithfully recreated with a given display type. Here's a short article about a 5 color per pixel
display that Sharp is developing, to improve color reproduction:
http://www.reghardware.com/2009/05/29/sharp_five_colour_lcd_panel/
- Atomic Spectra - started with the great demo: