| L5-01 OPTICAL BOARD - TOTAL INTERNAL REFLECTION |
| POWERFUL GREEN LASER POINTER |
| P3-31 X-RAY TUBE |
| M1-22 LASER DIFFRACTION - GRATINGS |
| L5-02 TOTAL INTERNAL REFLECTION IN LONG TANK |
| Special equipment ordered |
| A CD, A DVD, AND A VINYL RECORD |
| POWERFUL GREEN LASER POINTER |
| P3-67 FLUORESCENCE OF LAUNDRY SOAP |
| M4-02 NEWTON’S RINGS - PROJECTION |
| P3-71 VISIBLE LASER |
| M7-34 ROTATION OF POLARIZATION - POLAROID AND WAX PAPER |
| M7-05 ROPE AND COOKIE COOLERS |
| VIDEO CAM TO SHOW DIGITAL WATCH DISPLAY |
| N1-41 RAINDROP RAY MODEL |
| LITTLE HEADPHONE SPEAKER, SIGNAL GENERATOR, AND RADIO |
| K8-51 MICROWAVE OVEN |
| K8-52 MICROWAVE MAGNETRON |
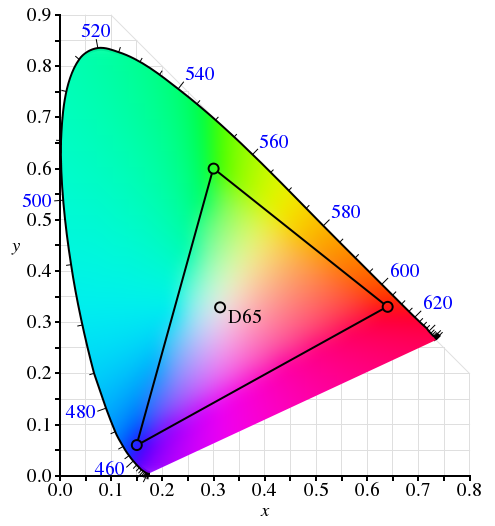
| N1-05 SPECTRA - VISIBLE AND INVISIBLE |
| AM
radio |
FM
radio |
Cell
phone |
Microwave
oven |
Visible |
|
| frequency |
550-1600 kHz |
87-106 MHz |
870-895 MHz |
2.45 GHz |
450-800 THz |
| wavelength |
300 m (1 MHz) |
3 m (100 MHz) |
30 cm (1 GHz) |
12 cm |
0.6 microns (500 THz) |
| bandwidth |
10 kHz |
200 kHz |
30 kHz |
| M9-03 CIRCULAR POLARIZATION - STICK MODEL |
| K8-42 RADIOWAVES - ENERGY AND DIPOLE PATTERN |
| K8-01 ELECTROMAGNETIC WAVE - MODEL |
| K8-03 LIGHT NANOSECOND |
| K3-03 DEMOUNTABLE TRANSFORMER - V VS N - PROJ METER |
| K7-61 TESLA COIL |
| K8-42 RADIOWAVES - ENERGY AND DIPOLE PATTERN |
| K8-01 ELECTROMAGNETIC WAVE - MODEL |
| K8-05 ELECTROMAGNETIC PLANE WAVE MODEL |
| K2-62 CAN SMASHER - ELECTROMAGNETIC |
| K2-43 LENZ’S LAW - PERMANENT MAGNET AND COILS |
| K2-02 INDUCTION IN A SINGLE WIRE |
| K2-61 THOMSON’S COIL |
| K2-44 EDDY CURRENT PENDULUM |
| RELAYS TO PASS AROUND THE ROOM |
| K1-03 FORCE ON CURRENT IN MAGNETIC FIELD |
| K2-01 EARTH INDUCTOR |
| K2-02 INDUCTION IN A SINGLE WIRE |
| K4-21 ST. LOUIS MOTOR |
| K4-41 MOTOR-GENERATOR PAIR |
| K5-12 BATTERY AND CURRENT - WORKING MODEL |
| K6-03 SERIES AND PARALLEL LIGHTS - BATTERY AND CLIP-ON WIRES |
| K6-01 SERIES AND PARALLEL LIGHTS - TWO BULBS |
| K6-03 SERIES AND PARALLEL LIGHTS - BATTERY AND CLIP-ON WIRES |
| J3-22 FARADAY CAGE - ELECTROSCOPE |
| K5-01 PIEZOELECTRICITY |
| J1-13 ELECTROSTATIC INDUCTION |
| J1-05 CHARGED BALLOONS |
| J1-12 INDUCTION - ELECTROSCOPE |
| J1-24 ELECTROSTATIC HAIR RAISING |
| J2-03 VAN DE GRAAFF GENERATOR WITH GROUND SPHERE |
| J2-14 LIGHTNING ROD SIMULATOR |
| J1-05 CHARGED BALLOONS |
| J1-21 ELECTROSTATIC ATTRAC AND REPULS - CHARGED CYLINDERS |
| K5-04 PIEZOELECTRIC GUN |
| H4-31 VIOLIN |
| H4-34 GUITAR AND OSCILLOSCOPE |
| H3-24 OPEN AND CLOSED PIPES |
| G2-01 MASS ON SPRING - HAND HELD |
| BOTTLES |
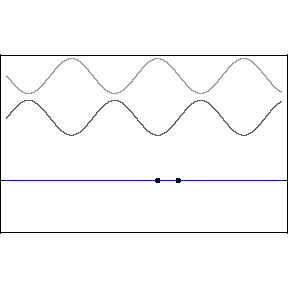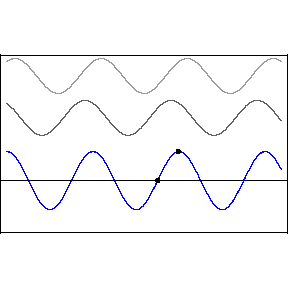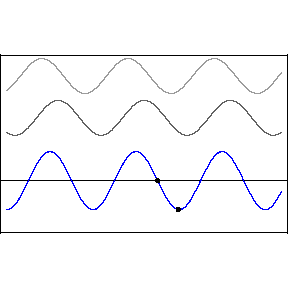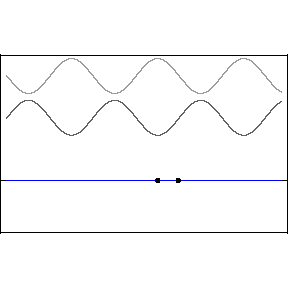
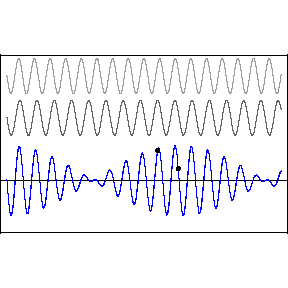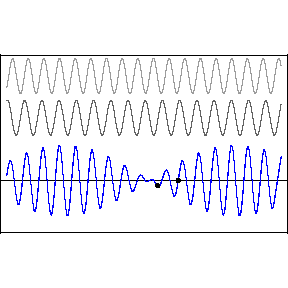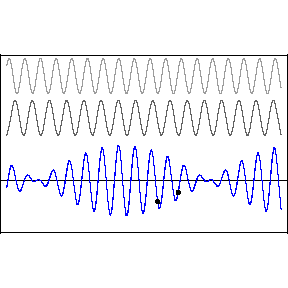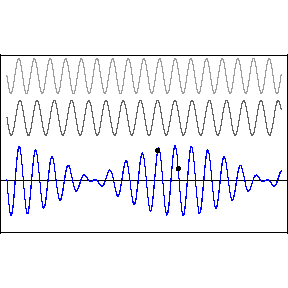
| (3/2)12 = 129.7, 27 = 128 |
| H4-34 GUITAR AND OSCILLOSCOPE |
| G2-01 MASS ON SPRING - HAND HELD |
| G3-28 SUSPENDED SLINKY |
| G1-15 PENDULA WITH 4 TO 1 LENGTH RATIO |
| G1-33 MASSES AND SPRINGS WITH SPIDER |
| C8-01 GIANT PENDULUM |
| I5-11 ADIABATIC PROCESS - AIR PISTON WITH THERMISTOR |
| I5-12 ADIABATIC EXPANSION OF AIR - FOG IN BOTTLE |
| I5-21 HEATING AIR BY COMPRESSION |
| I4-33 CRYOPHORUS |
| I4-19 CONDENSATION OF STEAM - SODA CAN COLLAPSE |
| THERMOMETER WITH LARGE DIGITAL READOUT, AND BEAKER OF WATER |
| I1-63 HYDROGEN EXPLOSION |
| I2-06 THERMOPILE WITH AUDIO OSCILLATOR |
| I4-52 CARBON DIOXIDE BALLOON ON LIQUID NITROGEN |
| I4-14 CHANGE OF STATE WITH BANG |
| C7-17 SUPERBALL |
| I3-33 HELIUM BALLOON ON LIQUID NITROGEN |
| I3-12 WATER BAROMETER - CAN CRUSHER |
| F1-01 FLUID PRESSURE VS. DEPTH |
| F1-04 EQUILIBRIUM TUBES |
| F1-06 WATER SEEKS ITS OWN LEVEL |
| F2-05 BUOYANCY - BOAT AND ROCK |
| F2-21 REACTION TO BUOYANT FORCE |
| HELIUM BALLOON |
| F1-11 HYDRAULIC PRESS |
| C6-11 SLIDING FRICTION - LECTURE TABLE AND FELT |
| RAMP, SKATEBOARD, AND LEAD BRICK |
| BICYCLE WHEEL |
| C8-21 ROCK AND WASTE BASKET |
| C3-05 INERTIA - PEN IN BOTTLE |
| C3-12 PENCIL AND PLYWOOD |
| C8-01 GIANT PENDULUM |
| A2-22 MAGNETIC VECTORS - LECTURE HALL |