| P4-01 GEIGER COUNTER |
| P4-08 IONIZATION SMOKE ALARM |
| M1-22 LASER DIFFRACTION - GRATINGS |
| M7-11 OPTICAL BOARD - BREWSTER’S ANGLE |
| L7-13 OPTICAL BOARD - GALILEAN TELESCOPE |
| L5-24 FIBER OPTICS - COMMUNICATION LINE |
| VINYL PHONO RECORD, CD, SMALL DVD |
| M8-01 POLAROIDS AND KARO SYRUP |
| P2-02 PHOTOELECTRIC EFFECT IN ZINC - ARC LAMP |
| P3-53 ATOMIC ENERGY LEVEL MODEL |
| P3-67 FLUORESCENCE OF LAUNDRY SOAP |
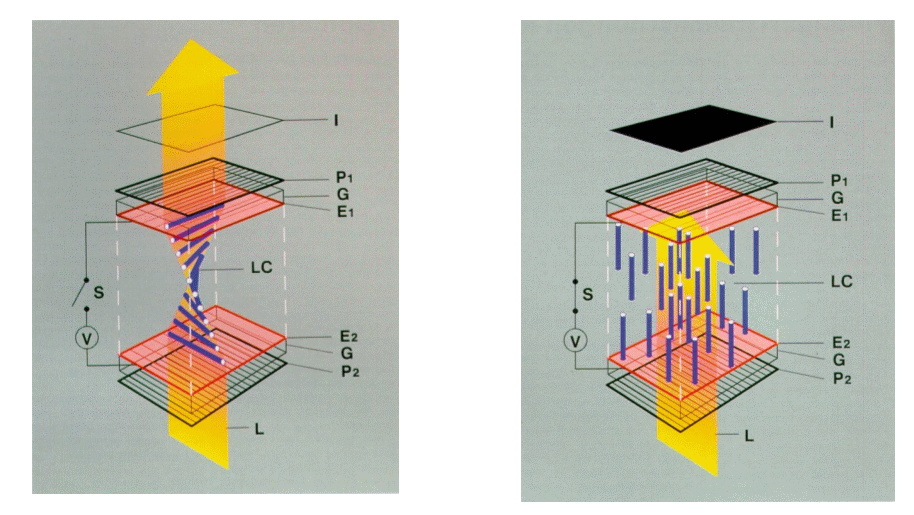
| N2-21 PRISMATIC SPECTRUM OF MERCURY - SUPERPRESSURE LAMP |
| N2-02 DIFFRACTION SPECTRA - THREE SOURCES - EXPENDABLE GRATINGS |
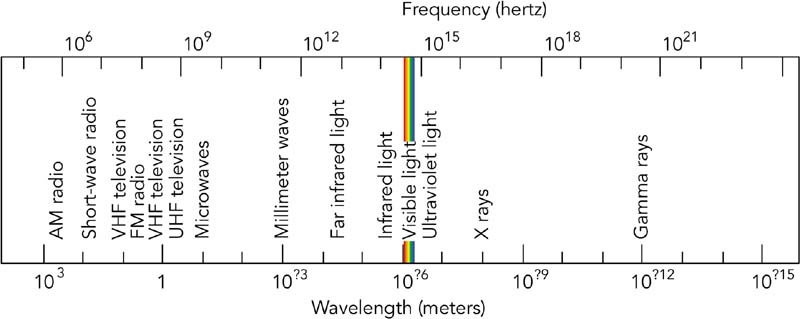
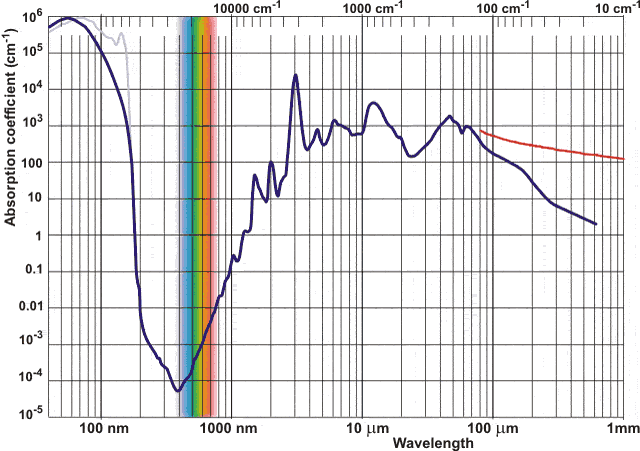
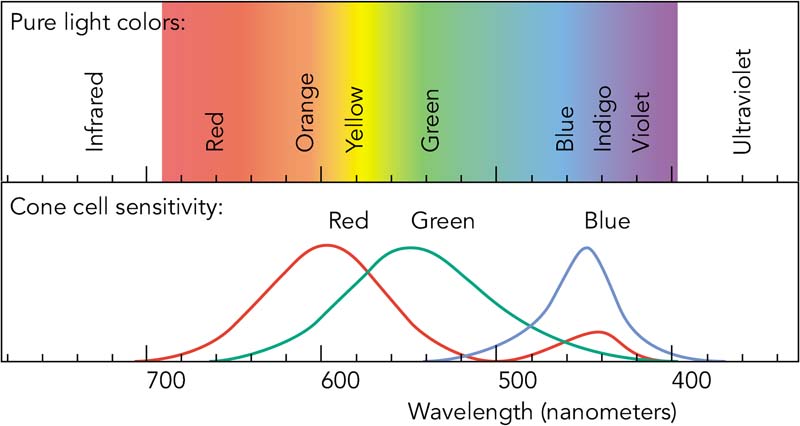
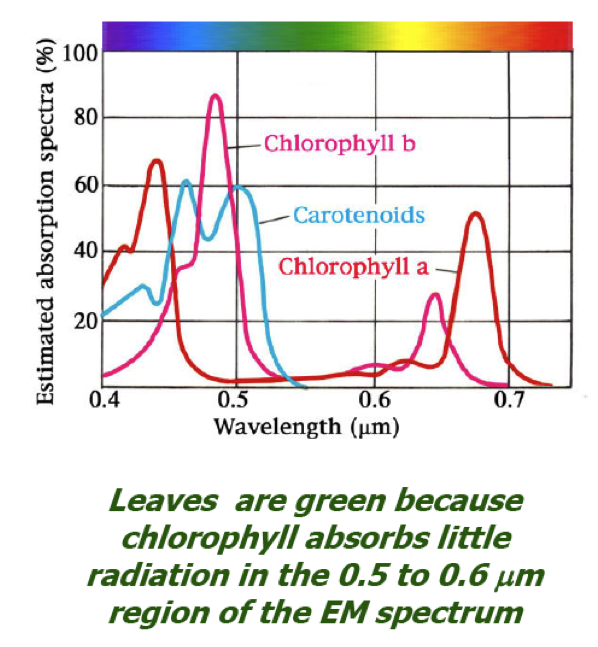
| N1-05 SPECTRA - VISIBLE AND INVISIBLE |
| N1-41 RAINDROP RAY MODEL |
| M7-05 ROPE AND COOKIE COOLERS |
| M7-34 ROTATION OF POLARIZATION - POLAROID AND WAX PAPER |
| K5-01 PIEZOELECTRICITY |
| K5-04 PIEZOELECTRIC GUN |
| K5-12 BATTERY AND CURRENT - WORKING MODEL |
| K5-32 RESISTANCE VS DIAMETER AND LENGTH |
| K5-36 RESISTORS AT LN TEMPERATURE - LIGHT BULB INDICATOR |
| G3-28 SUSPENDED SLINKY |
| G1-15 PENDULA WITH 4 TO 1 LENGTH RATIO |
| H4-31 VIOLIN |