2) A 500 g piece of copper is accidentally dropped into liquid nitrogen
(T = -180 oC). In order to warm up the copper, a student removes
it from the nitrogen and immediately places it in a Styrofoam cup containing
400 g of water. The water is at a temperature of 15 oC. Predict
the final equilibrium state that is attained within the Styrofoam cup.
Explain your reasoning and interpret, in words, the physical meaning of
each separate term that is present in any equation you use. Some of the
following constants may be of use: cwater = 1.0 cal/g oC; cice
= 0.49 cal/g oC; ccopper = 0.092 cal/g oC; Lf,water
= 80 cal/g; Lf,copper = 49 cal/g; k = 1.38 x 10-23
J/ oC 1 cal = 4.186 J
3) An experiment on the melting of ice is being done in an insulated
calorimeter set up so no heat is exchanged with the outside environment.
The calorimeter contains a mass m1 of water and a block of ice
having a mass m2, is floating in the water. Both the ice and
the water are at a temperature of 0 oC.
(a) What mass of boiling water, m3, must be added to the
system to produce only water at 0 oC? Express your answer in
terms of symbols, defining symbols for any heat capacities you require.
(b) Suppose m1 = 100 grams, m2 = 25 grams, and I
add 50 grams of boiling water. When the system comes to thermal equilibrium,
will there be any ice left? If there is none, what will the final temperature
of the water be? The following numbers may be of some use: 1 cal/gram-oC
80 cal/gram 540 cal/gram
4) For each of the following partial sentences, indicate whether they
are correctly completed by the symbol corresponding to the phrase greater
than (>), less than (<), or the same as (=).
(a) A chunk of iron is heated to a temperature of 120 oC.
It is then moved into a container of water at room temperature. The water
in the container has the same mass as the iron. The change in the water's
temperature due to the insertion of the iron will be __________ the change
of the iron's temperature due to its insertion into the water.
(b) A chunk of iron is heated to a temperature of 120 oC. It
is then placed into a hole prepared for it in a large block of ice. After
a period of time, the ice stops melting and the iron is sitting in a pool
of water melted out of the ice. The pool sits in a depression in the remaining
ice which it has melted out. The temperature of the water in that pool
is __________ 0 oC.
(c) A chunk of iron is sitting on a scale. It is then covered by a bell
jar which is air-tight and which has a nozzle connected to a vacuum pump.
When the air is pumped out of the bell jar, the scale reading will be __________
it was when there was air. (Assume the scale would read zero if nothing
were sitting on it, even when the air is pumped out.)
(d) A chunk of iron is sitting on a scale. The iron and the scale are then
both immersed in a large vat of water. After being immersed in the water,
the scale reading will be __________the scale reading when they were simply
sitting in the air. (Assume the scale would read zero if nothing were sitting
on it, even when it is under water.)
5) Three important concepts in the study of thermodynamics are, temperature,
heat, and internal energy. Discuss the meaning of these three concepts
being careful to distinguish between them.
6) I have two beakers of water. Each holds one kg of water. One is sitting
at room temperature (25 °C). The other is sitting on a hot plate and
is boiling. I place a block of iron with a mass of 0.2 kg into the boiling
water and leave it until the water starts boiling again. I then remove
it from the boiling water and place it into the other beaker. If the specific
heat of iron is 0.12 Cal/(kg-°C) what will be the final temperature
of the water and iron once they come to thermal equilibrium?
7) At time t=0 a hot object at a temperature T1 is placed
in an environment at a temperature T0. The temperature of the
object will be some function of time, T(t). This function will satisfy
the equation: 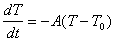
(a) Explain what this equation is telling you in words.
(b) Show that the function 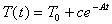 satisfies
the equation above.
satisfies
the equation above.
(c) Find the value of the constant c for the particular case we are considering.


![]()
![]() satisfies
the equation above.
satisfies
the equation above. ![]()