
Teaching Physics with the Physics Suite
Edward F. Redish
Home | Action Research Kit| Sample Problems | Resources | Product Information
Problems Sorted by Type | Problems Sorted by Subject | Problems Sorted by Chapter in UP
 |
Teaching Physics with the Physics Suite Edward F. Redish Home | Action Research Kit| Sample Problems | Resources | Product Information |
Problems Sorted by Type | Problems Sorted by Subject | Problems Sorted by Chapter in UP |
A. You’ve been talking about the concept of energy for many years – since grade school. You’ve probably had chemistry and biology classes in which energy was a central element of the conversation. Now in physics we are slowly building the concept of a particular kind of energy – mechanical energy (kinetic and potential). What do you think energy is? How would you explain it to a non-scientific roommate? To a seven year old? (Not just “mechanical energy” – all energy.)
A variety of interesting behaviors can be described with different shapes of potential energy. Four qualitatively different PE shapes are shown in the figure below. In each figure, the horizontal axis is a position and the vertical axis is a potential energy. You can think of them as frictionless tracks with a small ball rolling on them. The energy they describe is like a gravitational potential energy of the ball.
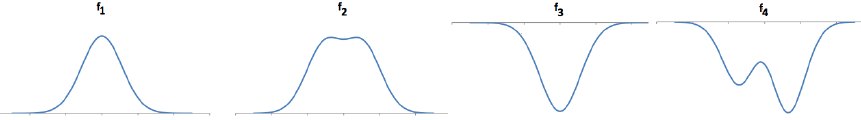
Some of these situations are analogous to the situation of chemical bonding. Consider the following examples and discuss which if any of the potential energy shapes provide a useful analogy. Explain why you think so. (The energy going into or coming out of a chemical reaction may be in the form of kinetic energy -- seen as heat -- or in terms of electromagnetic energy -- a photon.)
B. Two moles hydrogen atoms may interact and form an H2 molecule. When they do so, they release 103 Cal (kcal).
C. Decomposing one mole of water molecules (H2O) into its component atoms requires the input of 220 Cal.
D. The decomposition of one mole of water molecules into H2 and O2 molecules requires the input of 113 Cal.
E. The phosphorus in the head of a match burns in air, giving off energy when you strike the match.
Page last modified October 27, 2010: PE29