
Teaching Physics with the Physics Suite
Home | Action Research Kit| Sample Problems | Resources | Product Information
Problems Sorted by Type | Problems Sorted by Subject | Problems Sorted by Chapter in UP
 |
Teaching Physics with the Physics Suite
Home | Action Research Kit| Sample Problems | Resources | Product Information |
Problems Sorted by Type | Problems Sorted by Subject | Problems Sorted by Chapter in UP |
You are assigned the task of working with a desk-top sized magnetic spectrometer for the purpose of measuring the ratio of C12 to C14 atoms in a sample in order to determine its age.*
For this problem, let's concentrate on the magnet that will perform the separation of masses. Suppose that you have burned and vaporized the sample so that the carbon atoms are in a gas. You now pass this gas through an "ionizer" that on the average strips one electron from each atom. You then accelerated the ions by putting them through an electrostatic accelerator — two capacitor plates with small holes that permit the ions to enter and leave.
The two plates are charged so that they are at a voltage difference of DV Volts. The electric field produced by charges on the capacitor plates accelerate the ions to an energy of qDV. These are then introduced into a nearly constant, vertical magnetic field. (See the figure below.) If we ignore gravity, the magnetic field will cause the charged particles to follow a circular path in a horizontal plane. The radius of the circle will depend on the atom's mass. (Assume the whole device will be placed inside a vacuum chamber.)
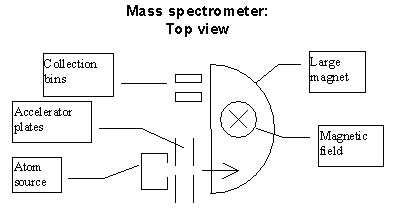
Answer three questions about how the device works.
(a) We would like not to use too high a voltage. If DV is 1000 Volts, how big magnetic field would we require to have a plausible "table-top-sized" instrument? Is this a reasonable magnetic field to have with a table-top sized magnet?
(b) Do the C12 and C14 atoms hit the collection plate far enough apart? (If they are not separated by at least a few millimeters at the end of their path we will have trouble collecting the atoms in separate bins.)
(c) Can we get away with ignoring gravity? (Hint: Calculate the time it would take the atom to travel its semi-circle and calculate how far it would fall in that time.)
Not finding what you wanted? Check the Site Map for more information.
Page last modified October 19, 2002: MG 02