The microcomputer can now be used as a tool in the laboratory by students of all ages. The ability to connect a device (a probe) to the computer that can measure things in the real world (such as temperature, position, sound intensity, pH, light intensity and force) now allows students and teachers to acquire information about the world in a way that is new and exciting and can make a major contribution to the science conceptual development of the user. The ability of the microcomputer to transform these data into a real-time graph as the experiment progresses is a second critical contribution to conceptual development. This personally-observed information from the real world can play a major role in honoring the constructivist view of learning that suggests that each person constructs his or her personal world view. A major factor in this process is the quality of each individual's interaction with physical systems and the personal effort expended to create explanations and understandings of a variety of science concepts rendered visible by the system. When the computer plays a role in this manner, it will be identified as a Microcomputer-Based Laboratory (MBL). The combination of the equipment and the computer programs required to enable the computer to serve as a laboratory device will be called probeware. This present description contrasts sharply with the limited description of the micro-computer as a laboratory instrument in New Trends in Physics Teaching (Layman, 1984).
Teachers in today's classrooms and students currently enrolled in our colleges and universities still have limited opportunities to develop fully their own set of science concepts and rarely have an opportunity to use the microcomputer in the laboratory in support of this. An MBL based program will be described that was designed for practicing middle school teachers and recommendations will be offered for the type of commitment that has to be made in the sciences and in science education by teachers involved in training and professional development activities.
The operation and usefulness of the computer in science concept development may be shown using probeware. One experiment, often used when working with middle-school teachers, involves the processes of heating and cooling. It is an experiment that has not been readily done in the past and takes full advantage of the dynamic nature of the computer's ability to display a live temperature v. time graph of two parts of a physical system as the experiment progresses. This allows observers of the experiment to begin making judgments about the experimental outcomes from the instant the experiment begins, a critical feature of MBL work.
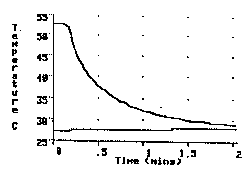
The experiment involves placing a 250 g copper cylinder, initially heated to near the boiling temperature of water, into a beaker containing 250 g of room temperature water and observing the temperature change of both the cylinder and the water bath over time. The observers of the experiment are asked to make two judgments before the experiment begins. The first is to estimate the time interval that should be chosen for the x axis of the graph, so that a complete record of the temperatures of the two parts of our system will be obtained. The second is to make a prediction of the final temperature reached by the cylinder and the water after they have been together for a long period of time. The most common prediction for the final temperature is a temperature half-way between the initial temperature of the cylinder and the water bath. The temperature history of both the cylinder and the water in an actual experiment is visible in Figure 1.

Fig. 1. Temperature v. time graph of a copper cylinder and equal mass of water as a hot cylinder (top line) is immersed in the cold water (bottom line)
Questions to be answered by the observers after the experiment are: Was the final temperature the value that you predicted? and what accounts for the great difference in the temperature response of the copper in contrast to the temperature response of the equal mass of water into which the copper was placed?
From the very beginning of the experiment, as shown in the graph, it becomes obvious that the water's temperature change is so slight that the usually predicted final temperature, midway between the initial temperature of the copper and the water, will not be correct. As the teachers realize the inaccuracy of their predictions, many will begin to offer reasons for the observed difference in the temperature changes. Some will immediately suggest that the cylinder gave up more heat energy than the water absorbed. This provides evidence that observer are offering explanations based on only temperature changes, a clear indication of an alternative conception in need of changing. Others however, will quickly point out that the cylinder is immersed in the water, so that all the heat energy transferred from the cylinder must acquired by the water.
As discussion among the teachers proceeds, most will finally recognize that all the heat energy lost by the copper was indeed transferred to the water. They also will recognize that the masses were the same an that the only thing remaining to account for the extreme difference in the temperature changes of the copper and the water is some fundamental difference in the character or behavior of copper and of water as they exchange heat energy. This leads to the opportunity to invent the concept of specific heat before invoking that term. If the observers have carefully followed the behavior of this system and can fully describe its behavior in their own words, they will possess a 'shared experience' that can serve as the basis for an operational definition of specific heat before the formal introduction of that term. This is a procedure describe most skillfully by Arnold Arons in his book on teaching introductory physics (Arons, 1990, p. 2).
New technologies such as MBL are thought of as something that will of direct benefit to students, without the realization that we as teachers may benefit as well. This tool should first be used with the teachers in their pre-service and in-service programs, and used in a way that brings about conceptual change in the teachers. After recognizing its role in their own personal experiences of conceptual change, teachers also can recognize its power to help effect conceptual changes in their students.
The majority of the studies of concepts of temperature and heat (also most other concepts) are carried out with children. Procedures are described for detecting students' pre-conceptions and for bringing about conceptual change through activities in the classroom and laboratory, sometimes using modern technology such as the microcomputer. This focusing of alternative conception detection and remediation on children implies that it is only the students who lack appropriate conceptual knowledge of temperature and heat and not their teachers. Perhaps we have only ready access to students rather than to teachers when monitoring conceptual states. It also may reflect the common assumption that if the textbooks describe these concepts properly and if we have designed appropriate laboratory work for our students, then this is sufficient. Our work with teachers would suggest otherwise.
The University of Maryland Middle School Probeware Project was designed as a two-summer workshop series for middle-school teachers. It provided training and experience in the use of the microcomputer as a laboratory tool and in the procedures to be followed in their middle-school physical science classes. We estimate that during the 1990-91 school year, two years after the last summer workshop, there were sixty teachers and approximately 5,000 middle school students from Washington D. C. and three of its suburbs in the states of Maryland and Virginia who used microcomputers in their laboratory work on the concepts of temperature and heat.
The project provided an introductory MBL workshop the first summer. This was followed by an intervening school year during which teachers and students began using MBL. An advanced MBL works took place in the second summer. This was followed by a second year which the teachers continued using MBL in their classrooms, but with a new group of students. The project was designed to measure teacher and student concepts of temperature and heat, and the teacher's knowledge of how to teach these concepts.
Twenty-two middle-school science teachers from three large urban school districts, representing thirteen different schools, took part in the project. The project centered around using MBL to teach heat energy and temperature concepts. Throughout both workshops teachers performed a variety of laboratory activities related to heat and temperature using MBL. The laboratory activities required teachers to make predictions, do experiments using MBL to test their predictions and carry out careful post-experiment discussions. This process was to play a role in the teacher's own concept development and also provide a model of the procedures to be used by their students. Teachers also spent time designing and testing experiments to be used with their students.
Semi-structured interviews were used to probe teachers' understandings of heat and temperature concepts (content knowledge) as well as their understanding of how to teach these concepts (pedagogical content knowledge) (Shulman, 1986 and 1987). Eight randomly-selected teachers participated in a full set of five interviews during the project period. The first interview was conducted during the initial week of the introductory workshop, the second interview at the beginning of the semester following the introductory workshop, the third interview after the following spring semester just before the advanced workshop, the fourth interview at the beginning of the fall following the advance workshop and the last interview after each teacher's second year of using MBL with students. Interviews lasted between 20 and 40 minutes depending upon the responses made by the teacher. Teachers were asked to respond to the following tasks:
The verbal data generated in the interviews were analyzed using concept propositional analysis and content analysis. Analysis of the data shows that before the MBL workshop the middle-school teachers held alternative concepts related to heat and temperature. For example, seven of the eleven teachers participating in the pre-introductory workshop interviews believed that temperature was a measure of heat energy. After the introductory workshop, eight of the eleven teachers held satisfactory scientific views of temperature. In spite of this improvement in the concepts of temperature, many teachers still held alternative concepts or had incomplete ideas regarding heat energy. Most teachers exhibited poor understanding of how to teach heat energy and temperature concepts both before and after the introductory workshop. A complete record of the conceptual states of the teachers was the goal. Additional interviews were conducted with teachers after their first year of using MBL with students, after the advanced workshop offered during the second summer and at the close of the academic year following the advanced workshop. Preliminary processing of the full set of interviews shows that some teachers made conceptual progress on temperature concepts during the first summer workshop, some after the year of working with their students and some only after the second workshop.
All teachers finished the program with a good understanding of temperature and the pedagogical procedures for helping students with their concepts of temperature. This cannot be said for concepts of heat energy which proved much more intractable. Some teachers never developed the concept of heat energy to a point where they could carefully describe what they would accept from their students as suitable answers to the distinction between heat energy and temperature. Nor were they able to suggest the critical experiments that should be done by their students to develop a deep conceptual distinction between these two concepts. Some finally recognized that the two were different, but could not fully articulate these differences, usually due to a limitation in their concept of heat energy.
These results appear very consistent with the conceptual change literature and Shulman's (1986 and 1987) work on pedagogical content knowledge. They seem to provide an indication that helping teachers form appropriate scientific concepts and develop rich conceptual knowledge of how to teach science concepts and how to use new technology in the classroom requires careful work over a prolonged period, beginning during courses taken in the science departments, continue in science methods courses and require only minor adjustment in in-service programs after they have begun to teach. It should not be relegated to special in-service projects undertaken long after they have begun teaching, as happened with the UMPP project.
The UMMPP project experience suggests several recommendations for the design of in-service programs. A two-summer workshop program with follow-up activities and support in the intervening and following years is the minimum required to produce lasting change in the conceptual base and teaching skills of teachers. Monitoring the conceptual state of each teacher at the various junctions of the program and providing conceptual state feedback to the teachers (which the UMMPP project did not do) may offer an important improvement in the approach. It is also appropriate to focus more sharply on teaching strategies that might conceivably arise out of the personal conceptual development of the teachers.
The UMMPP experience with classroom teachers shows that they are appreciative of the opportunity to finally understand science concepts that were previously textbook bound and studied in a classical manner when they took science in their undergraduate courses. In-service teachers must be given the opportunity to wrestle with personal conceptual change, because it rarely occurs in their regular science courses. Years of teaching may provide teachers with insights into dealing with students but may not be fully supported by the necessary range of appropriate science concepts. As shown earlier, this conceptual development must become a shared responsibility of both the science departments and the science education groups in colleges and universities. This sharing of responsibility also requires improved communication and collaboration between the sciences and science education.
There is certainly the need for a full survey of teachers' conceptual understanding in all areas of science, at the various levels of teaching, and within countries that have different avenues for' the preparation of teachers. It is not satisfactory to simply measure student progress and ignore the conceptual state of the teachers.
We have had the opportunity to make presentations and conduct workshops dealing with MBL in Thailand, Mexico, Italy and the Philippines. Often those attending the presentations and workshops lacked direct access to the microcomputers and the probeware required to carry out MBL. In spite of this, we believe it is important for teachers to understand and recognize the major role that the computer could play in their own and their students' conceptual development. Computers will become available in all parts of the world and it is important for teachers to consider the computer as a key element in their classroom and laboratory work.
A subset of our teachers who have very limited preparation in science are those who teach in elementary schools. It would be valuable for these teachers to have had the personal experience of using microcomputers in some of their science coursework and to recognize the role that the computer can play in helping elementary students as well.
One cannot forget that the computers that provide laboratory support for students can also provide support for the many tasks to be carried out by teachers, such as keeping grades, monitoring equipment inventories, providing word processing support for their classroom documents and many others. The curriculum work carried out by teachers in the workshops was done in word processing and stored on disks so that the final exercises provided for their students could be modified by the teachers to reflect the special needs within their classrooms.
Microcomputer-based laboratories are a new technology and their introduction into schools requires special support. School districts need to supply two levels of support. The first is technical support for the computers and their maintenance, and for the probeware that makes the experiments possible. The second is leadership. The most effective leaders in the school districts are those who have participated in the workshop. This enables the leaders to be at or above the level of the teachers' conceptual and pedagogical content knowledge, and to fully understand the operation of the equipment. Teachers experiencing difficulties using this technology will quickly stop if they do not receive timely support.
The UMMPP program also required a minimum of two teachers from any one middle school to provide a critical mass within each school. In addition, teachers using a new technology must continue to meet as a group if curriculum changes are to occur within the school district.
Towards the end of the introductory workshop a single day workshop, designed by the workshop participants, was given for administrators from each participating school as well as for the science education leaders in the school district. This workshop provided a level of understanding of the value of probeware and helped to ensure support in the schools when the teachers began implementing the program. Sometimes the local school leaders also controlled the sources of funds with which to purchase probeware and general laboratory equipment, so their participation and understanding was critical.
This chapter has argued that training and professional development programs for primary and secondary school teachers of science and technology should involve the microcomputer as a tool for the teacher's own content knowledge and pedagogical content knowledge development. It also should serve as a personal productivity tool while they are involved in pre-service or in-service programs.
It must, however, be recognized that this is such a new tool that time and effort will have to be devoted to bringing the microcomputer into an effective role in the life of the science teacher and the students in their classrooms.
Arons, A. B. 1990. A Guide to Introductory Physics Teaching. New York, John Wiley & Sons.
Layman, J. W. 1984. Microcomputers as Laboratory Instruments. In: E. J. Wenham (ed.), New trends in Physics Teaching, Vol. IV, pp. 297-300. Paris, UNESCO.
Shulman, L. S. 1986. Those who Understand: Knowledge Growth in Teaching. Educational Researcher, Vol. 15, No. 2, pp. 4-14.
_____ 1987. Knowledge and Teaching- Foundations of the New Reform. Harvard Educational Review, Vol. 57, No. 1, pp. 1-22.
 U. of Md. Physics Education Activities HomePage
U. of Md. Physics Education Activities HomePage
 U. of Md. Physics Education Research Group HomePage
U. of Md. Physics Education Research Group HomePage
 U. of Md. Computers in Physics Education HomePage
U. of Md. Computers in Physics Education HomePage