Studying Real World Nano-bots with Optical Tweezers

By: Arthur La Porta
Anyone who follows the news or is a fan of Star Trek is familiar with the notion that sometime in the distant future mankind will make frequent use of ‘nano-probes.’ When introduced to the body these tiny robots, which are usually depicted as tiny metallic gadgets, will perform incredibly sophisticated tasks, tugging on cellular structures, manipulating DNA, destroying invading viruses, etc. The only thing that makes this notion science fiction rather than science fact is the strange appearance of the nano-probes and the assumption that they will be relegated to the distant future. In fact, nature is replete with nano-probes and nano-bots that perform functions no less astonishing than what is depicted in any science fiction movie. However, these nano-bots are not metallic gadgets, but single molecule molecular motors, that hydrolyze ATP (or other nucleotide tri-phosphates) and convert the energy released to mechanical work.
These single molecule biological motors perform a variety of functions, such as transporting cargo throughout the cell, helping to pull pairs of chromosomes apart during cell division, or copying and editing genomic information stored in our DNA. The specific molecular motor that I am most interested in is RNA polymerase. This molecule crawls along a DNA molecule, prying the two strands apart so that one strand can be used as a template for creating a complementary RNA molecule. It moves on the DNA in discrete 1 nucleotide steps, ligating an additional RNA nucleotide to the chain at each position. The ‘messenger’ RNA molecule is subsequently sent to the ribosome, which uses it to synthesize a protein needed in the cell.
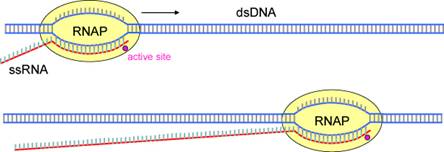
Figure 1. Schematic representation of RNA polymerase (RNAP) transcribing a double
stranded DNA molecule, creating a single stranded RNA chain.
RNA polymerase has been extensively studied using a technique called “optical tweezers.” In these experiments a tiny latex sphere is attached to the RNA polymerase molecule transcribing DNA in solution and the piece of DNA being copied is tacked down to the bottom of the sample chamber. The latex sphere is captured by an optical trap, which exerts a constant tension on the DNA tether. By observing the motion of the sphere, the position of RNA polymerase on the DNA molecule is measured as a function of time. In this way, we can observe a gene being transcribed in real time. As a result of such studies, we can observe the kinetics of RNA polymerase as it moves down the template and see how individual molecules react to signals that are encoded in the DNA sequence. These signals might cause RNA polymerase to halt, or to become unstable and dissociate from the template. This information is critical to understanding the role of RNA Polymerase in regulating the production of proteins in a cell. Future work at the University of Maryland may involve determining how RNA Polymerase reacts to torque as well as force, and determining how other proteins may interact with RNA Polymerase to change its behavior.
xxxxxxxxxxxxxxxxxxxxxxxxx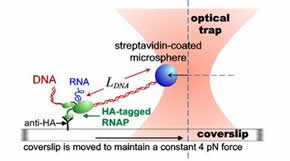
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxFigure 2 Schematic representation of the use of optical tweezers
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx to study RNA polymerase.
Dr. La
Porta is an assistant professor of Physics for the University of Maryland and IPST. He is a member of the Biophysics research group. Feel free to contact him at, alaporta@umd.edu .
|