
Problems for
Intermediate Methods in Theoretical Physics
Edward F. Redish
 |
Problems for |
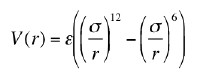
Discuss, using a graph of the original and approximate functions, what we hope to accomplish by choosing to do this. To get the best approximation in the neighborhood of the minimum of the potential, what should we choose for V0, k and r0?
| University of Maryland | Physics Department | Physics 374 Home |
|---|---|---|
 |
 |
 |
Last revision 6. September, 2004.