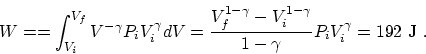(a) First of all, the internal energy change is easy to
calculate since it only depends on the temperature change. (
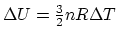 .) For
.) For
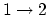 process, since the
volume does not change, the work is zero, and so
process, since the
volume does not change, the work is zero, and so
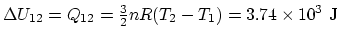 . For
. For
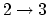 , there is no
exchange of heat (adiabatic)
, there is no
exchange of heat (adiabatic) 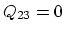 which means that the work
which means that the work
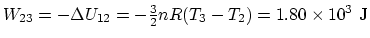 .
For
.
For
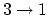 ,
,
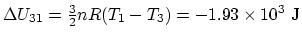 . From the differential relation between the
volume variation and the internal energy for a constant-pressure
process,
. From the differential relation between the
volume variation and the internal energy for a constant-pressure
process,
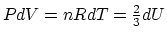 ,
,
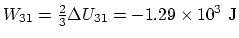 . Finally,
. Finally,
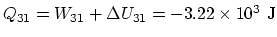 .
In the overall process, if you add all the numbers above,
.
In the overall process, if you add all the numbers above, 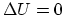 ,
,
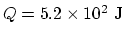 , and
, and
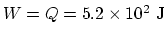 .
.
(b) The initial volume is
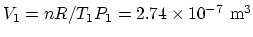 .
At point 2, since the volume is the same as at point 1,
.
At point 2, since the volume is the same as at point 1,
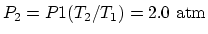 . At point 3, since the pressure is the same
as at point 1,
. At point 3, since the pressure is the same
as at point 1,
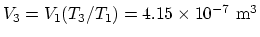 .
.
![]() .
At point 2, since the volume is the same as at point 1,
.
At point 2, since the volume is the same as at point 1,
![]() . At point 3, since the pressure is the same
as at point 1,
. At point 3, since the pressure is the same
as at point 1,
![]() .
.