-
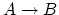
- The internal energy increases (+) since the value
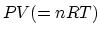 increases and the internal energy is only proportional to
the temperature. The work is positive (+) since
increases and the internal energy is only proportional to
the temperature. The work is positive (+) since 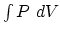 is positive in
this process. From
is positive in
this process. From
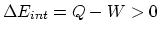 , we find
, we find 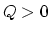 (heat +).
(heat +).
-
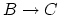
- The internal energy increases (+) since the value
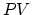 increases. The work is zero due to no volume change (0). In this
kind of isovolumetric processes,
increases. The work is zero due to no volume change (0). In this
kind of isovolumetric processes,
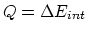 . Therefore,
. Therefore, 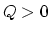 (+).
(+).
-
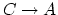
- The internal energy decreases (-) for the
reason explained above. The work is negative because the volume decreased.
From
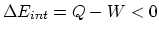 and
and 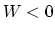 , we find that
, we find that 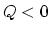 .
. - 5-5
- From
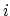 to
to 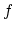 , the path-independent internal energy
increase is
, the path-independent internal energy
increase is
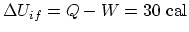 . (a) Along
. (a) Along 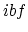 ,
,
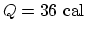 . So
. So
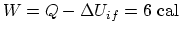 . (b) For
. (b) For 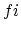 ,
,
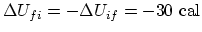 and
and
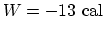 . So
. So
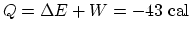 . (c) Since
. (c) Since
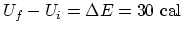 ,
,
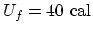 . (d) For
. (d) For 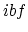 , the only work is done over
, the only work is done over 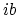 since
since 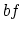 is an isovolumetric process,
so
is an isovolumetric process,
so
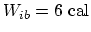 . Since we are given that
. Since we are given that
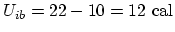 ,
,
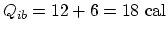 . For
. For 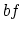 ,
,
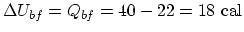 .
.
- 5-6
- Let mass of the piston
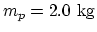 , area of the
cylinder
, area of the
cylinder
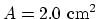 , atmospheric pressure
, atmospheric pressure
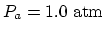 , density of the steam
, density of the steam
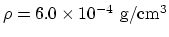 , the
latent heat of vapourisation
, the
latent heat of vapourisation
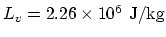 , and the
rate of contraction
, and the
rate of contraction
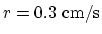 .
Then the total pressure on the steam is
.
Then the total pressure on the steam is
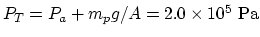 .
(a) Neglecting the volume of water compared to that of steam per unit
mass, the volume contraction (condensation) rate is
.
(a) Neglecting the volume of water compared to that of steam per unit
mass, the volume contraction (condensation) rate is 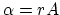 , and
the mass condensation rate is
, and
the mass condensation rate is
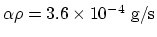 .
(b) Heat loss occurs through the loss of the latent heat:
.
(b) Heat loss occurs through the loss of the latent heat:
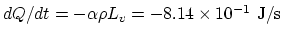 .
(c) The work rate is
.
(c) The work rate is
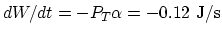 . Therefore, the
internal energy change rate is
. Therefore, the
internal energy change rate is
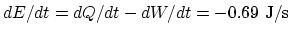 .
.
- 5-7
- The power transfer per unit area is
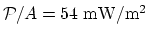 , and the thermal conductivity is
, and the thermal conductivity is
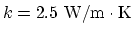 .
Since
.
Since
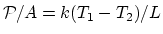 , at
, at 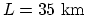 and
and
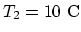 ,
,
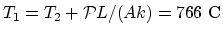 .
.
- 5-8
- From
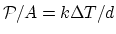 ,
(a) for a glass,
,
(a) for a glass,
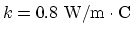 , and the temperature
difference is
, and the temperature
difference is
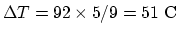 . So the heat
loss rate is
. So the heat
loss rate is
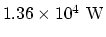 .
(b) From the formula
.
(b) From the formula
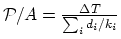 ,
for a glass
,
for a glass
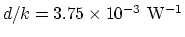 and that of the air gap
of
and that of the air gap
of 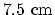 is
is
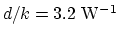 . So the thermal
insulation is mostly done by the air-gap.
. So the thermal
insulation is mostly done by the air-gap.
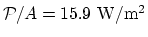 .
.
- 6-1
- (a) Here the temperature is
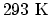 and
and
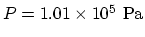 . From
. From 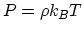 , where
, where 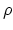 is the number density,
is the number density,
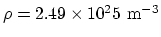 . (b) Molar mass of
. (b) Molar mass of 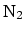 : 28 g.
Molar mass of
: 28 g.
Molar mass of 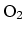 : 32 g. If 25 % is oxygen and
75 % is nitrogen, the average molar mass is
: 32 g. If 25 % is oxygen and
75 % is nitrogen, the average molar mass is 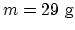 . So the total mass
is
. So the total mass
is
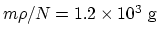 .
.
- 6-2
- From the impulse-momenta relationship,
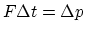 . When one molecule hits the wall, the momentum
transfer to the wall is twice the initial momentum normal to the wall,
because the normal component of the momentum of the molecule is
reversed in sign. The initial normal momentum of an individual molecule:
. When one molecule hits the wall, the momentum
transfer to the wall is twice the initial momentum normal to the wall,
because the normal component of the momentum of the molecule is
reversed in sign. The initial normal momentum of an individual molecule:
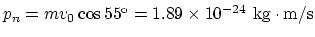 . Since
. Since
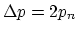 , and
there are
, and
there are 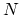 such molecule that hits the area
such molecule that hits the area 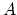 per second,
per second,
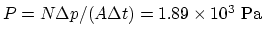 .
.
- 6-3
- Average translational kinetic energy:
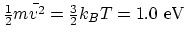 . This corresponds to
. This corresponds to
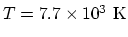 .
.
- 6-4
- The molar mass:
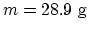 . Molar specific heat:
. Molar specific heat:
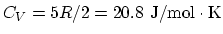 . (a) Since one mole is
. (a) Since one mole is 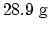 , the constant volume specific heat per unit mass is
, the constant volume specific heat per unit mass is
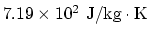 . (b) Mass of the air: From
. (b) Mass of the air: From 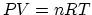 ,
we find that there is 28 mol of air, which corresponds to 0.81 kg. (c) From
,
we find that there is 28 mol of air, which corresponds to 0.81 kg. (c) From
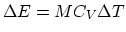 , we need
, we need
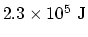 of energy.
(d) If the piston is free to move, the air will move in such a way
that the pressure is constant. Since the ration
of energy.
(d) If the piston is free to move, the air will move in such a way
that the pressure is constant. Since the ration 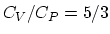 , we
need energy input of
, we
need energy input of
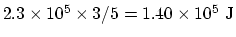 .
.
- 6-5
- Let
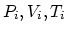 be the initial values of the pressure, volume and
temperature, and
be the initial values of the pressure, volume and
temperature, and 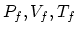 be the final values. (a) From the
relation for an adiabatical process
be the final values. (a) From the
relation for an adiabatical process
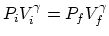 ,
the final pressure is
,
the final pressure is
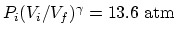 .
(b) From
.
(b) From 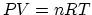 , we find that
, we find that
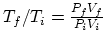 , so
, so
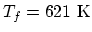 .
.
(b) Firstly, we determine that the sign of the work done by the gas is
negative since ![]() over the process. The value of the work
is simply the area enclosed by the process in the
over the process. The value of the work
is simply the area enclosed by the process in the ![]() diagram:
diagram:
![]() .
.
Next: About this document ... Up: Homework Solutions for PHYS262, Previous: Homework Solutions for PHYS262, Hyok-Jon Kwon
2001-08-08