


Next: Heat Engine, Entropy, and
Up: Homework Problems for PHYS262,
Previous: Heat and the First
- 6-1
- (a) What is the number of molecules per cubic metre in air at
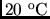 and at pressure of 1.0 atm (
and at pressure of 1.0 atm (
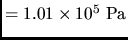 )? (b) What
is the mass of this
)? (b) What
is the mass of this 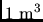 of air ? Assume that 75 % of the
molecules are nitrogen (
of air ? Assume that 75 % of the
molecules are nitrogen (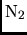 ) and 25 % oxygen (
) and 25 % oxygen (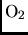 ).
).
- 6-2
- The mass of the
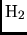 molecule is
molecule is
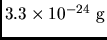 . if
. if 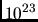 hydrogen molecules per second strike
hydrogen molecules per second strike 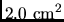 of
wall at an angle of
of
wall at an angle of 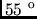 with the normal when moving with a
speed of
with the normal when moving with a
speed of
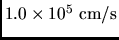 , what pressure do they exert on the
wall?
, what pressure do they exert on the
wall?
- 6-3
- At what temperature is the average translational kinetic
energy of a molecule equal to
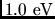 ? (
? (
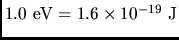 ).
).
- 6-4
- A vertical cylinder with a heavy piston contains air at 300
K.The initial pressure is 200 kPa, and the initial volume is
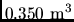 . Take the molar mass of air as
. Take the molar mass of air as
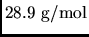 and assume
that
and assume
that 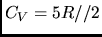 . (a) Find the specific heat of air at constant
volume in units of
. (a) Find the specific heat of air at constant
volume in units of
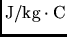 . (b) calculate the mass of the
air in the cylinder. (c) suppose the piston is held fixed. Find the
energy input required to raise the temperature of the air to 700 K.
(d) Assume again the condition of the initial state and that the heavy
piston is free to move. Find the energy input required to raise
temperature to 700 K.
. (b) calculate the mass of the
air in the cylinder. (c) suppose the piston is held fixed. Find the
energy input required to raise the temperature of the air to 700 K.
(d) Assume again the condition of the initial state and that the heavy
piston is free to move. Find the energy input required to raise
temperature to 700 K.
- 6-5
- A mass of gas occupies a volume of 4.3 L at a pressure of
1.2 atm and a temperature of 310 K. It is compressed adiabatically to
a volume of 0.76 L. Determine (a) the final pressure and (b) the final
temperature, assuming it to be an ideal gas for which
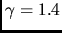 . ( Hint: it is not necessary to make any unit conversions.)
. ( Hint: it is not necessary to make any unit conversions.)
- 6-6
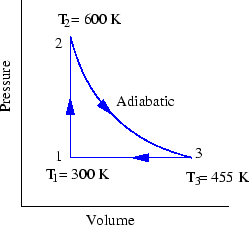
- A reversible heat engine carries 1.00 mol of an ideal
monatomic gas around the cycle shown in Fig. 10. Process
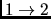 takes place at constant volume, process
takes place at constant volume, process
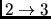 is adiabatic, and process
is adiabatic, and process
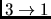 takes place at a
constant pressure. (a) Compute the heat
takes place at a
constant pressure. (a) Compute the heat 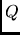 , the change in internal
energy
, the change in internal
energy 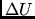 , and the work done
, and the work done 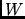 , for each of the three
processes and for the cycle as a whole. (b) If the initial pressure
at point 1 is 1.0 atm, find the pressure and the volume at points 2 and 3.
, for each of the three
processes and for the cycle as a whole. (b) If the initial pressure
at point 1 is 1.0 atm, find the pressure and the volume at points 2 and 3.
Figure 10:
Prob 6-6
 |
- 6-7
- The escape speeds from the surface of the earth and the moon are
respectively 11.2 km/s and 2.3 km/s.
(a) Compute the temperatures at which the rms speed is
equal to the speed of escape from the surface of the earth for
molecular oxygen. (b) Do the same for the moon. (c) The
temperature high in the earth's upper atmosphere is about 1000
K. Would you expect to find much hydrogen there? Much oxygen?
- 6-8
- At 273 K and
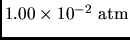 the density of a
gas is
the density of a
gas is
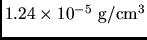 . (a) Find
. (a) Find 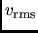 for
the gas molecules. (b) Find the molecular mass of the gas and identify it.
for
the gas molecules. (b) Find the molecular mass of the gas and identify it.



Next: Heat Engine, Entropy, and
Up: Homework Problems for PHYS262,
Previous: Heat and the First
HJK
2001-07-22