


Next: The Kinetic Theory of
Up: Homework Problems for PHYS262,
Previous: Temperature
- 5-1
- Calculate the specific heat of a metal from the following
data: a container made of the metal has a mass of 3.6 kg and contains
14 kg of water. A 1.8 kg piece of the metal initially at a
temperature of
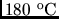 is dropped into the water. The container and
water initially have a temperature of
is dropped into the water. The container and
water initially have a temperature of 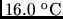 and the final temperature
of the entire system is
and the final temperature
of the entire system is 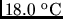 .
.
- 5-2
- In a solar water heater, energy from the sun is gathered
by rooftop collectors, which circulate through tubes in the
collector. The solar radiation enters the collector through a
transparent cover and warms the water in the tubes; this water is
pumped into a holding tank. Assuming that the efficiency of the
overall system is 20% (that is, 80% of the incident solar energy is
lost from the system), what collector area is necessary to take water
from 200-L tank and raise its temperature from 20 to
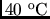 in 1.0 h.
The intensity of incident sunlight is
in 1.0 h.
The intensity of incident sunlight is
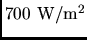 .
.
- 5-3
- What mass of steam at 100 C must be mixed with 150 g of
ice at 0 C, in a thermally insulated container to produce liquid water
at
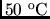 ?
?
- 5-4
- A thermodynamic system is taken from an initial state
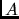 to another
to another 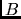 and back again to
and back again to 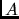 , via state
, via state 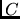 , as shown by the
path
, as shown by the
path 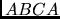 in the
in the 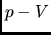 diagram of Fig. 7a.
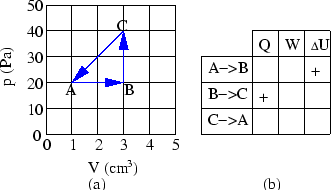
(a) Complete the table in Fig. 7b
by filling in appropriate
diagram of Fig. 7a.
(a) Complete the table in Fig. 7b
by filling in appropriate  or
or  indications for the signs of the
thermodynamic quantities associated with each process. (b) Calculate
the numerical value of the work done by the system for the complete
cycle
indications for the signs of the
thermodynamic quantities associated with each process. (b) Calculate
the numerical value of the work done by the system for the complete
cycle 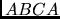 .
.
Figure 7:
Prob 5-4
 |
- 5-5
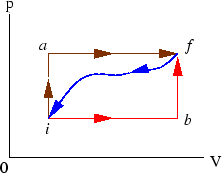
- When a system is taken from state
 to state
to state 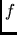 along
the path
along
the path 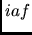 in Fig. 8, it is found that
in Fig. 8, it is found that 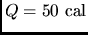 and
and
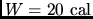 . Along the path
. Along the path
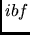 ,
, 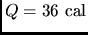 . (a) What is
. (a) What is 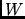 along the path
along the path 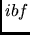 ? (b)
If
? (b)
If 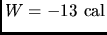 for the curved
return path
for the curved
return path 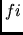 , what is
, what is 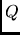 for this path? (c) Take
for this path? (c) Take
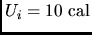 . What is
. What is
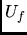 ? (d) If
? (d) If
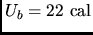 , what are the
, what are the 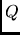 values for process
values for process
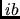 and process
and process 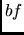 ?
?
Figure 8:
Prob 5-5
 |
- 5-6
- A cylinder has a well fitted 2.0-kg metal piston whose cross
sectional area is
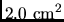 (Fig. 9).
The cylinder contains water and steam at
constant temperature. The piston is observed to fall slowly at a
rate of
(Fig. 9).
The cylinder contains water and steam at
constant temperature. The piston is observed to fall slowly at a
rate of 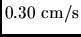 because heat flows out the cylinder through
the cylinder
walls. As this happens, some steam condenses in the chamber. The
density of the steam inside the chamber is
because heat flows out the cylinder through
the cylinder
walls. As this happens, some steam condenses in the chamber. The
density of the steam inside the chamber is
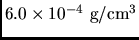 and the atmospheric
pressure is 1.0 atm. (a) Calculate the rate of condensation of the
steam. (b) At what rate is heat leaving the chamber? (c) What is the
rate of change of internal energy of the steam and water inside the
chamber?
and the atmospheric
pressure is 1.0 atm. (a) Calculate the rate of condensation of the
steam. (b) At what rate is heat leaving the chamber? (c) What is the
rate of change of internal energy of the steam and water inside the
chamber?
Figure 9:
Prob 5-6
 |
- 5-7
- The average rate at which heat flows out through the
surface of the earth in North America is
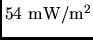 , and the average thermal
conductivity of the near surface rocks is
, and the average thermal
conductivity of the near surface rocks is
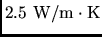 . Assuming a surface
temperature of
. Assuming a surface
temperature of 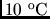 , what should be the temperature at a depth of 35
km (near the base of the crust)? Ignore the heat generated by the
presence of radioactive elements.
, what should be the temperature at a depth of 35
km (near the base of the crust)? Ignore the heat generated by the
presence of radioactive elements.
- 5-8
- (a) What is the rate of heat loss in watts per square
meter through a glass window 3.0 mm thick if the outside temperature
is -20 F and the inside temperature is +72 F? (b) A storm window is
installed having the same thickness as the glass but with an air-gap of
7.5 cm between the two windows. What will be the corresponding rate
of heat loss presuming that conduction is the only important heat loss
mechanism?



Next: The Kinetic Theory of
Up: Homework Problems for PHYS262,
Previous: Temperature
HJK
2001-07-22